
Healthcare has finally entered its digital era, leaving behind piles of paper that once moved between patients and doctors or within an organization. However, with the freedom of becoming digital comes the great responsibility of managing valuable and sensitive data. You need to ensure data integrity, security, and full compliance with healthcare standards. Keep reading this article to find out how you can ensure full GxP compliance in your facility!
What is GxP in healthcare organizations, and why does it matter
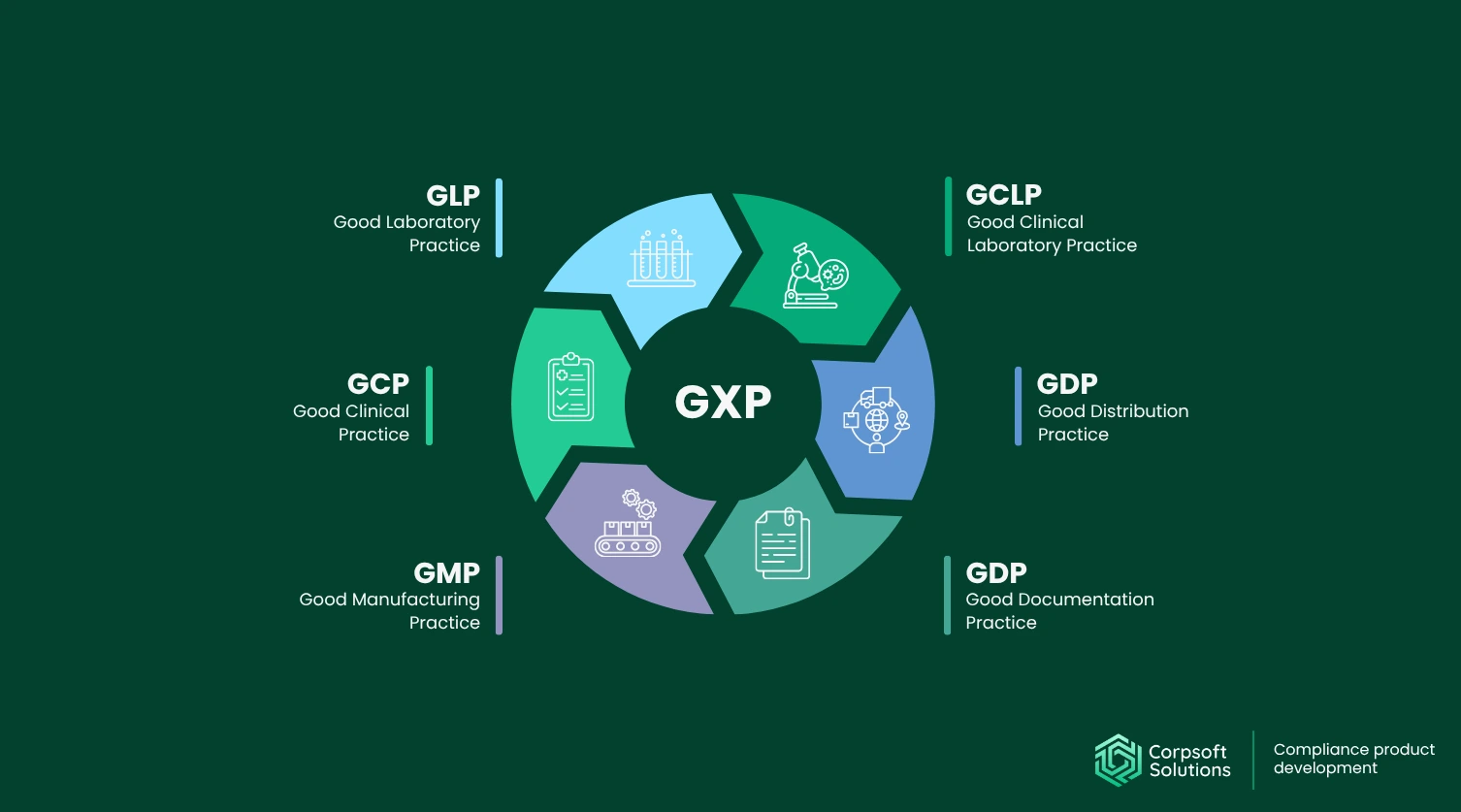
GxP stands for Good “X” Practice, which is a family of rules (the “X” changes depending on the domain) designed to make sure healthcare products and services are safe, reliable, and traceable.
Speaking of real-life, GxP meaning translates to an umbrella term that collects sector-specific expectations and other important guidelines, such as ISO 9001 or ISO 17025. By using GxP, organizations can ensure proper execution of tests and clinical trials, maintain high-quality drug manufacturing, and guarantee that products are stored, transported, and documented in compliance with the latest standards.
While there are dozens of GxP umbrellas, there are 6 core standards:
- GLP (Good Laboratory Practice). There are rules for non-clinical laboratory studies. Here, facilities stick to the rules to show that their results are reliable and reproducible.
- GCP (Good Clinical Practice). These are ethical and scientific standards for clinical trials involving human subjects. Here, you need to have informed consent from every patient, ensure protocol compliance, and data integrity.
- GMP (Good Manufacturing Practice). It ensures that medicinal products and devices stick to the latest quality standards, such as contamination control and batch records.
- GCLP (Good Clinical Laboratory Practice). It’s a bridge between GLP and GCP for labs involved in clinical testing.
- GDP (Good Distribution Practice). These are requirements for storage, transport, and distribution of medicines that help facilities preserve quality across the supply chain.
- GDP (Good Documentation Practice). By using it, you can be sure that your record tracking is flawless.
As of the mid-2020s, GxP frameworks are applied across healthcare to maintain complete traceability, streamline digital systems, and safeguard data integrity in every aspect of healthcare operations.
Where is GxP typically used?
Currently, GxP practices are an essential part of almost every healthcare-related process. If you want to ensure that you stick to all regulations, you need to implement them into your workflow.
But where exactly can you implement a GxP standard? To save you time, we’ve gathered the most common usage cases in one table.
|
Full Name of GxP practice |
Where It’s Used |
Real-World Example |
| Good Laboratory Practice | Research and diagnostic laboratories | A diagnostic lab uses it to ensure all tests are performed according to validated methods and recorded for traceability. |
| Good Clinical Practice | Clinical trials and human studies | A hospital conducting a new oncology drug trial must comply with GCP to protect patients’ rights and ensure reliable clinical data. |
| Good Manufacturing Practice | Drug and medical device manufacturing | A hospital’s in-house pharmacy compounding medications must follow GMP to ensure each preparation meets safety and purity standards. |
| Good Clinical Laboratory Practice | Clinical testing environments | A laboratory that processes patient samples uses it to align both clinical and analytical procedures with regulatory expectations. |
| Good Distribution Practice | Storage and transportation | A distributor of temperature-sensitive vaccines applies GDP standards to show that their product is taken proper care throughout the entire supply chain. |
| Good Documentation Practice | All healthcare operations | A medical center uses it to ensure that every patient record, lab result, and approval form is properly dated, signed, and securely stored. |
| Good Storage Practice | Warehouses and hospital storage areas | A hospital’s storage facility follows it to control humidity and temperature for sensitive drugs and diagnostic materials. |
Keep in mind that your businesses should emphasise data integrity, as it’s the only way to pass audits. If they see that you don’t care about clients’ safety, be prepared to face hefty fines. Remember that you need to stick to the ALCOA+ rule, where data must be
- Attributable, so you can identify who performs a specific action.
- Legible, as all actions are readable and clear.
- Contemporaneous, meaning that the system documents actions right when they are occurring.
- Original, as the platform needs to store and edit the original version, without overburdening the cloud with dozens of extra copies.
- Accurate, as each document should be error-free and faithfully reflect your activity.
- Completeness, with the “nothing should be missing” rule.
- Consistency, where all records must follow a logical order with timestamps.
- Endurance, as all records should be stored on durable media (typically, secured cloud solutions) and be securely protected.
- Availability, so you can easily retrieve data during audits or inspections.
If you don’t follow all these criteria, you are risking failing the GXP qualification and facing regulatory fines.
GxP in the сontext of ISO and FDA: How they connect
When we talk about compliance in healthcare, GxP doesn’t exist in isolation. It works hand-in-hand with ISO standards and FDA regulations to create a solid framework for quality, safety, and traceability.
ISO standards like ISO 9001 (Quality Management Systems) and ISO 13485 (Medical Devices) are basically a roadmap for keeping quality across your organization. They guide your team on how to design work processes and constantly improve them.
This way, you can demonstrate to auditors that your organization adheres to even the strictest ISO guidelines. At the same time, GxP certification shows that your facility aligns directly with FDA regulations, such as 21 CFR Part 11 (electronic records), 21 CFR Part 210/211 (pharmaceutical manufacturing), and 21 CFR Part 820 (medical devices).
In practice, GxP + ISO + FDA compliance creates a triple layer of protection:
- International quality standards (ISO) set the structure and management expectations.
- GxP principles ensure those expectations are actually carried out in labs, clinics, and manufacturing.
- FDA regulations define enforceable rules and provide the regulatory teeth, including audits, inspections, and approvals.
If your organization works globally, GxP ensures that you meet both FDA and ISO requirements. It reduces duplication and makes your business adhere to the latest regulations. The regular GXP compliance training helps reduce audit findings and keeps your team confident and prepared.
Common challenges in GxP compliance
As a rule, healthcare organizations are struggling to stay compliant. But they are usually overburdened due to complex processes, fragmented systems, and outdated workflows.
To address these gaps, more companies are turning to GxP compliance services and consultants that help them identify risks, validate systems, and streamline documentation across departments. The first step to fix this problem is to understand what hinders your GxP compliance.
Let’s examine the most common obstacles that organizations face on their way to compliance, and how professional GxP consulting can help overcome them.
Fragmented systems
One of the most common challenges uncovered during GxP audits is the use of fragmented digital systems. Many healthcare organizations rely on different software for different parts of their workflow, where one tool is for patient records, another for lab management, and yet another for administrative tasks.
It often happens with any clinic and lab. Such fragmentation also increases the risk of errors, slows down audits, and makes it hard to trace any issue back to its source.
Imagine a hospital where the lab tracks patient test results in one system, the pharmacy records are stored in another, with no possibility of integration under a single dashboard, while clinical trials are managed in spreadsheets.
It means that staff must manually pull data from multiple sources. It delays the audit process and increases the chance of errors, potentially triggering legal warnings.
Manual record-keeping
Another common challenge that our team meets in GXP compliance consulting is that many healthcare facilities still prefer manual record-keeping. When organizations rely on paper logs, spreadsheets, or other manual methods to track processes, there will be mistakes.
Even the best doctors and nurses can make errors due to exhaustion. Records can be misplaced, incomplete, or even misinterpreted. It creates compliance gaps and slows down the facility’s workflow. For instance, a trial coordinator can manually record patient visits on paper. If even one page goes missing, an FDA auditor may flag the clinic, which can lead to legal consequences.
To minimize such risks, you need to move from manual record-keeping to automated, validated digital systems. Implementing GxP computer systems validation ensures that every electronic record and process meets the latest regulatory standards. It helps you centralize and automate record management, reduce errors, speed up audits, and provide a reliable, traceable history of every process.
Audit complexity
Audits are always stressful. But if you have inconsistent or scattered records, they may become a nightmare. In this case, you may face a complex GxP auditing with slow approvals and postponed launches.
Let’s imagine that you are a representative of a hospital’s in-house pharmacy and an FDA inspector asks you to provide batch records for compounded medications. But all our documentation is scattered across spreadsheets, so staff spend hours reconciling the information. The delay raises concerns for the auditors, and you’ll receive additional follow-up requests and corrective action requirements.
Audit complexity is a clear sign that your processes may need better integration, documentation, and automation. Streamlined systems and digital tools make audits smoother, faster, and less risky.
High cost and low reliability of manual monitoring
Keeping track of both quality and compliance manually might seem manageable. Meanwhile, in reality, even in small clinics, administrators are drowning in routine. Every manual check, data entry, or report takes hours of human work. Also, keep in mind that the most careful people also make mistakes.
Over time, this manual approach becomes too expensive and unreliable. Worse, it can lead to critical compliance gaps, inaccurate reporting, or even product quality issues that directly affect patient safety.
Let’s imagine that you are a pharmaceutical manufacturer that relies on staff to manually log temperature and humidity data for storage facilities. One missed entry or an incorrect reading during a weekend shift goes unnoticed, and you need to throw off the whole batch of medications.
That’s why you need to automate compliance checks and quality monitoring. With the right digital tools and professional GxP services, you can track every parameter, detect deviations, and create reports within a few clicks.
Lack of process control
When departments use separate systems, it becomes impossible to maintain full visibility. In such situations, data often gets siloed, resulting in poor communication. In the long run, it results in situations when you discover that your facility has some recurring issues only after they cause damage.
For example, let’s say your R&D team updates a formulation, but the production floor isn’t immediately informed. It results in inconsistent batches, rework, and compliance deviations that could have been easily prevented with integrated process control.

Process control is a multi-level process, and you need to ensure consistency, accuracy, and traceability at every stage.
Without a unified system, you can’t trace issues back to their root cause or ensure that corrective actions are applied consistently. In highly regulated industries, this lack of end-to-end visibility is a risk to compliance and product quality.
Complex data management and process oversight
Even the smallest clinic dealt with thousands of records, from equipment calibration logs to employee training and batch release documentation. If this data is scattered across multiple systems or stored in outdated formats, GxP validation becomes a nightmare.
A single outdated document can trigger non-compliance during an audit. Without a centralized data infrastructure, tracking process performance and identifying recurring issues takes far more time than it should.
For example, if laboratory results, CAPA records, and supplier audits are managed in different tools, it’s almost impossible to get a real-time view of overall quality performance. Teams waste hours cross-verifying data instead of improving processes.
By implementing an integrated quality management system, organizations can gain full visibility into their operations and streamline data flow. At the same time, they can be sure that every piece of information is always audit-ready.
Impact on operational efficiency and patient trust
When your internal processes are poorly managed, the effects ripple far beyond compliance checklists. First of all, your team may spend hours or even days on fixing errors and redoing tasks that should’ve been automated. It will result in delayed batch releases, postponed clinical trial milestones, and reduced operational efficiency.
In the long-term perspective, it will result in the loss of patient trust. Any quality lapse can damage your reputation. In severe cases, the damage can be so significant that it may take years to recover your status as a reputable healthcare organization.
How software solutions support GxP compliance in healthcare
Ensuring GxP compliance may take a lot of time, as these regulations cover every aspect of a healthcare or pharma organization. The huge amount of data, high regulatory stakes, and need for end-to-end process oversight can make anyone anxious. That’s why you need to automate as many processes as possible to successfully pass GXP compliance certification.
Where you can use digital solutions in healthcare and pharma
Today, almost every aspect of a clinic or healthcare institution is digitalized. As we’ve seen in previous sections, manual data handling typically ends badly.
That’s why digital tools are now widely used in:
- Clinical trials and research. You no longer need to rely on paper reports, as you can use electronic data capture (EDC) systems, while risk-based monitoring tools help prioritize high-risk areas or patients, reducing oversight.
- Laboratory and diagnostic processes. Leverage Lab Information Management Systems (LIMS) to track samples, tests, equipment calibration, and deviations in real time.
- Pharmaceutical manufacturing and compounding. In such situations, healthcare institutions benefit from batch record automation, which ensures every step in drug manufacturing follows GMP standards. Meanwhile, environmental monitoring systems can track temperature, humidity, and other critical parameters across storage and production.
- Distribution and supply chain. To make the distribution smoother, adopt advanced inventory management platforms. These systems track every shipment, monitor storage conditions, and ensure GDP compliance in real time.
- Administrative and quality management. A good QMS centralizes SOPs, training records, CAPA processes, audits, and documentation.
Each software addresses specific aspects of GxP compliance. Risk management software helps you identify weak points, while a document management system keeps all files in one place. Audit and reporting tools gather all data together, analyze it, and give you in-depth insights.
Artificial intelligence takes this one step further. AI-powered tools can predict potential deviations even before they happen, automatically review SOPs for inconsistencies, and detect anomalies. By incorporating these tools, you take a proactive approach.
What to choose: custom vs off-the-shelf solutions
When it comes to choosing GXP compliance services and tools, you have two options: off-the-shelf or custom-built platforms. Off-the-shelf systems are ready-made, but they typically can cover only generic workflows. While they are quicker to launch, they are falling short for those who need to create a unique workflow.
Healthcare and pharmaceutical organizations have highly specialized operations, from clinical trials and lab workflows to multi-site manufacturing and distribution. That’s why off-the-shelf products rarely fit into the healthcare industry.
You need specific solutions to unlock the full potential of your health facility. A tailored platform perfectly fits your workflow, so you can automate even the most complex tasks and track everything from a single dashboard.
One of the key parts of GxP compliance is Computer Systems Validation (CSV). This means making sure that all digital systems handling regulated data must work correctly, reliably, and be safe. In simple terms, it’s proof that your software does exactly what it’s supposed to do, which is automatically manage and analyze vast amounts of data to ensure that you meet all regulatory requirements.
In this context, fully custom digital tools may perfectly suit your specific conditions. You can start by developing a minimum viable product (MVP) and then gradually refine it over time. This approach helps balance speed and customization, providing your team with a solution tailored to your workflows and regulatory requirements while maintaining GxP compliance.
One of our latest projects is a scalable telemedicine platform for remote patient care. By using it, doctors can consult patients and work with their clinical data even if the patient is thousands of miles away from the doctor.
This custom software solution provides maximum security while ensuring the clinic can easily scale its operations.
Conclusion
GxP has become a core part of a healthcare organization’s quality culture. However, it takes a lot of effort. You need to handle a huge amount of data and use the latest digital systems to ensure that they track even the slightest changes and patterns, while securely storing every bit of information.
Personnel tend to overwork, which leads to overlooking crucial details. This negligence may result in legal fines. Meanwhile, the automated system is always monitoring every process, ensuring regulatory compliance and catching the smallest deviations before they escalate into bigger issues.
By leveraging proven platforms and implementing centralized, secure, and interoperable digital solutions, healthcare and pharmaceutical organizations can maintain high-quality standards, protect patient safety, and stay audit-ready.
The Corpsoft Solutions team helps healthcare and pharmaceutical organizations build digital ecosystems that meet international standards, ensure FDA and ISO compliance, and support ongoing quality management.
Ready to start your organization’s digital transformation and achieve GxP compliance? Partner with us to make it happen.
Subscribe to our blog


